2. What Concentration Of Salt Is Isotonic To Animal Cells (1 Pts)?
Osmosis Lab
Introduction: Human blood, at 0.9% common salt concentration, is a little less salty than seawater, which has a salt concentration of about 35 parts per thousand (iii.5%). If nosotros take seawater as an example of a solution, the salt is called the solute (the particles that are dissolved) and the water is the solvent (the liquid that dissolves the particles). Osmosis is the move of a solvent across a semi-permeable membrane from an surface area of lower solute concentration to an surface area of higher solute concentration. The water (the solvent) can move beyond the membrane merely the dissolved solutes (the sodium and chloride ions that class salt) cannot. In such situations, water will movement across the membrane to balance the concentration of the solutes on both sides. Cells tend to lose h2o (their solvent) in hypertonic environments (where in that location are more solutes outside than inside the prison cell) and gain water in hypotonic environments (where at that place are fewer solutes exterior than within the jail cell). When solute concentrations are the same on both sides of the prison cell, there is no internet water motility, and the jail cell is said to be in an isotonic environs. In this lab we volition exam samples of irish potato tissue to run across how much water they blot or release in table salt solutions of varying concentrations. This gives the states an indirect mode to measure the osmotic concentration inside living cells.
Hypo=under, iso=equal, hyper=over
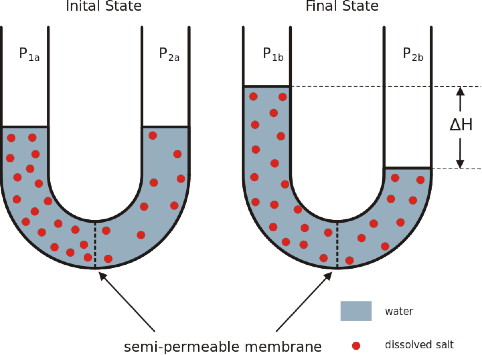
Compare initial and final states. Which way did the water move? Why?
- electronic residual (0.01 thousand range)
- metric ruler with mm scale
- metric measuring cups
- 6 cereal bowls or shallow pans
- a small piece of raw irish potato to cut into vi ~5 mm cubes
 (this square is 5 x five mm)
(this square is 5 x five mm) - single edged razor or pocketknife
- paper towels
- watch or clock
- tabular array common salt, distilled or tap water
- 6 beakers (250 ml or larger) or cups
Methods:
- Pre-mix 6 beakers of salt solutions (0%, 0.1%, 0.five%, 1%, two.v%, five%) in distilled water. You lot can apply this solution calculator to aid you brand your solutions. Just enter the h2o book of your container and the percentage of table salt you desire and it will tell you how many grams of salt to add. A 1% salt solution is i office salt to 100 parts water. To make a 1% salt solution, you could utilize a 100 ml bottle, add exactly 1 gram of salt (utilise your electronic balance) to your canteen, and bring the water volume upwards to 100 ml. To make a 0.1% solution, add 1 gram of common salt to 1000 ml of h2o (or add 0.i grand common salt to 100 ml of water). If you have more water than you demand, just stir well then discard the backlog.
- Prepare 6 small spud cubes with no skin that are all about equal in size (approximately five millimeters in length, width and acme) and absorb them dry on a paper towel. (Blot means just gently remove the surface water; no demand to squeeze them!)
- Mass (weigh) each to the nearest 0.01 grams, keeping them separate, and record each initial mass in Table ane. Don't wait also long before putting them into the solutions, as evaporation will occur.
- Fill each basin with one of the half dozen stock solutions, keeping rails of which is which! Characterization them. You won't be able to tell the salinity just past looking. Note which irish potato piece went into which basin.
- Exit i of the white potato slices in each of the table salt solutions for up to 24 hours so that they may gain (or lose) water by osmosis. (Keep them all in the common salt water the same amount of time--leaving them overnight is probable to requite the best results).
- Remove the slices, blot them dry on a paper towel, carefully re-weigh them and tape in the information table as final mass.
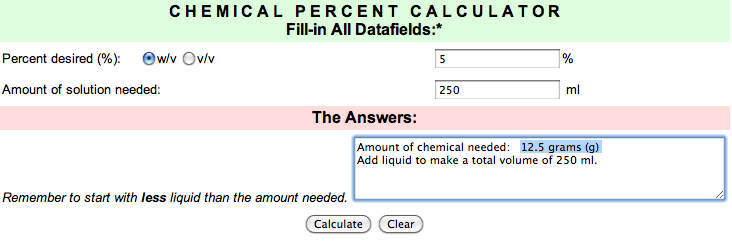
Click here to get to the calculator page, and thanks to the University of Oklahoma for this useful tool!
Results:
1. Record your bodily results in a table like this one:
| | | | | |
| Sample 1 | 0.0% | |||
| Sample 2 | 0.one% | |||
| Sample 3 | 0.5% | |||
| Sample 4 | 1.0% | |||
| Sample 5 | ii.5% | |||
| Sample 6 | 5.0% |
Table i: Changes in potato mass as a result of immersion in salt solutions.
2. Prepare a graph showing change in mass as a part of % table salt. Scale the ten-axis of your graph in units of 0.5 per centum. The y-axis has a null line half way up, indicating whether the samples lost or gained weight. You will take to scale the y-axis co-ordinate to your greatest and smallest changes in mass. Download this ![]() Excel spreadsheet if you lot need help making a graph.
Excel spreadsheet if you lot need help making a graph.
Figure 1: Modify in mass of potato (g) due to water gain/loss equally a function of salt concentration.
3. When completed, use a ruler to draw a straight line of all-time fit through your vi data points, or use the computer to graph your data and calculate the line of best fit. Where the line of best fit crosses the horizontal zero line, describe a vertical line down to the x-axis. This is the point at which the tater is isotonic with its surroundings, and is therefore the estimated salt concentration of the spud.
Questions:
- Why did some irish potato samples gain water and others lose h2o? Was at that place any pattern?
- When you lot drew the best fit line through your data and dropped the vertical line to the x-axis, what common salt concentration did you obtain (Guess if it is betwixt numbers)? What does this mean for the white potato?
- Why can't we use seawater to gargle our crops?
- What happens when a thirsty person drinks salt h2o to try to quench their thirst?
- Why does salted popcorn dry your lips?
- What happens to a jail cell'southward h2o when the exterior liquid is saltier than its interior?
- What happens to water outside the cell when the interior is saltier than its surroundings?
- When a jail cell gains water, what happens to its size and weight?
- When a cell loses water, what happens to its size and weight?
- When you put limp celery stalks in water, they house up. Why?
- Challenge question: Saltwater fish are hypotonic (less salty) to their surroundings while freshwater fish are hypertonic (more salty) to their surroundings. Assuming the salt can't motility, what must each fish practice with its fluids in order to compensate for the difference in salinity between the body and the surrounding surround?
Source: https://www2.nau.edu/lrm22/lessons/osmosis/osmosis.html
Posted by: shackelfordgremnecelues.blogspot.com

0 Response to "2. What Concentration Of Salt Is Isotonic To Animal Cells (1 Pts)?"
Post a Comment